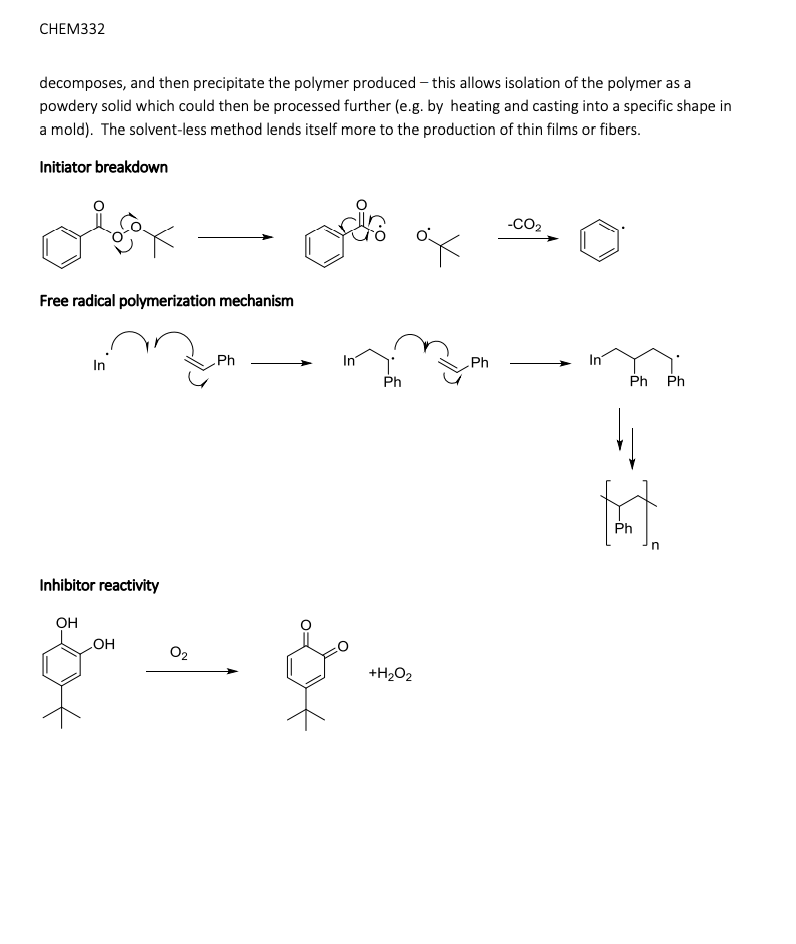
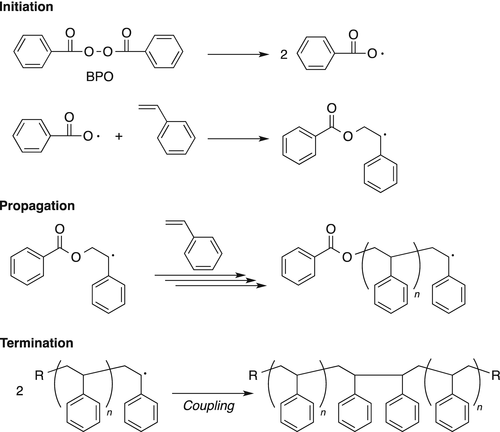
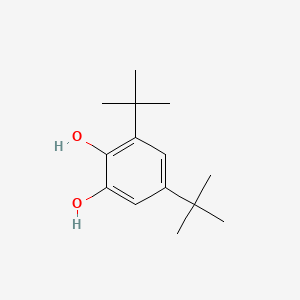
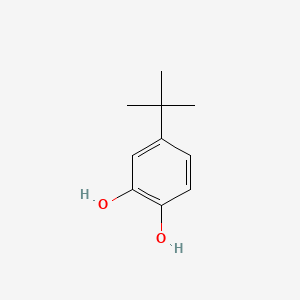
The influence of 4‐tert‐butylcatechol on the emulsion polymerization of styrene - Kemmere - 1999 - Journal of Applied Polymer Science - Wiley Online Library

Investigation of the electro-methoxylation reaction: Part 1. Electrochemical study of 4-tert-butylcatechol and 3,4-dihydroxybenzaldehyde in methanol - ScienceDirect

The influence of 4‐tert‐butylcatechol on the emulsion polymerization of styrene - Kemmere - 1999 - Journal of Applied Polymer Science - Wiley Online Library
![Polymerization Inhibitors | [Synthesis & Materials]Products | Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation Polymerization Inhibitors | [Synthesis & Materials]Products | Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation](https://labchem-wako.fujifilm.com/us/category/images/00209_img01.jpg)
Polymerization Inhibitors | [Synthesis & Materials]Products | Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation

Mechanistic study of electrochemical oxidation of 4-tert-butylcatechol: A facile electrochemical method for the synthesis of new trimer of 4-tert- butylcatechol - ScienceDirect
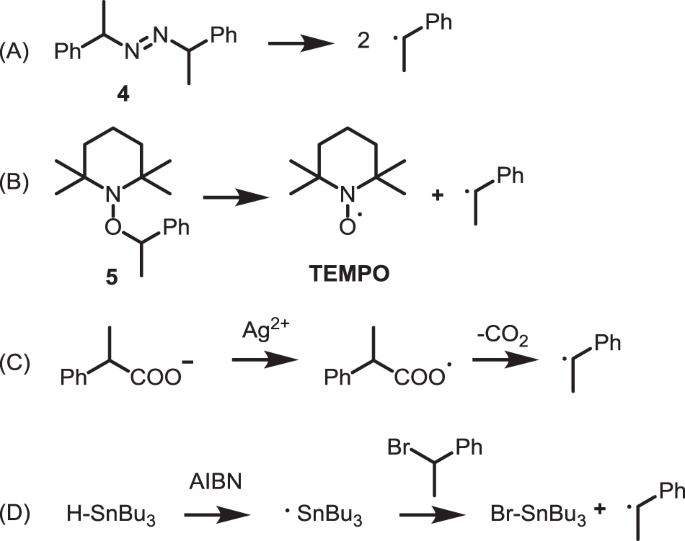
Polymerization inhibition mechanism of 1,4-naphthoquinone by experimentation and DFT calculations | Polymer Journal

Peroxy Radical Activated Addition of tert-Butylcatechol to 2,6-Di-tert-butyl-7-Substituted Quinone Methide Polymerization Retarders | Organic Process Research & Development

Peroxy Radical Activated Addition of tert-Butylcatechol to 2,6-Di-tert-butyl-7-Substituted Quinone Methide Polymerization Retarders | Organic Process Research & Development

Electrochemical study of 4-tert-butylcatechol in the presence of 1,3-dimethylbarbituric acid and 1,3-diethyl-2-thiobarbituric acid. Application to the electro-organic synthesis of new corresponding spiropyrimidine derivatives - ScienceDirect
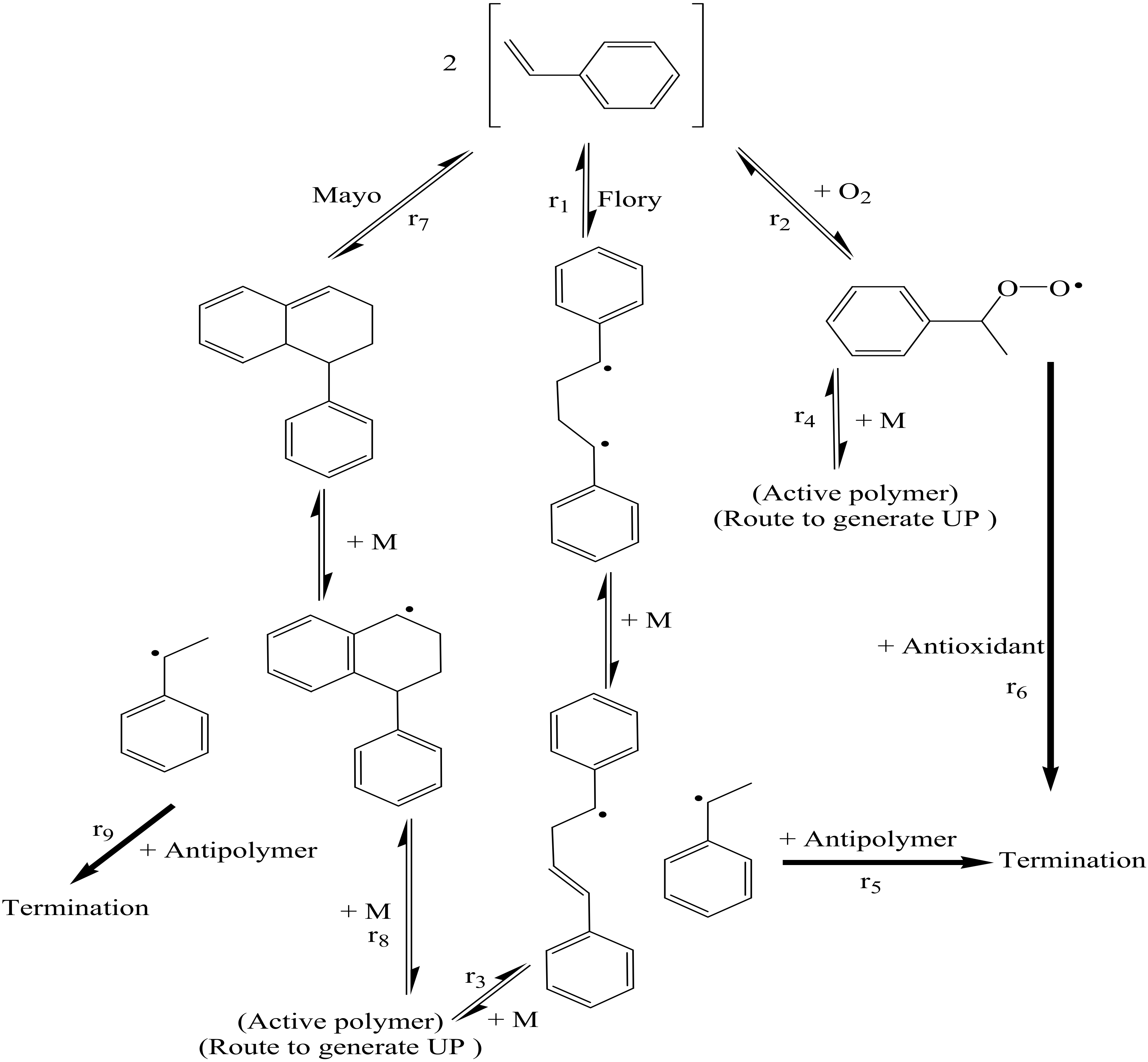
Processes | Free Full-Text | A Theoretical and Experimental Study for Screening Inhibitors for Styrene Polymerization
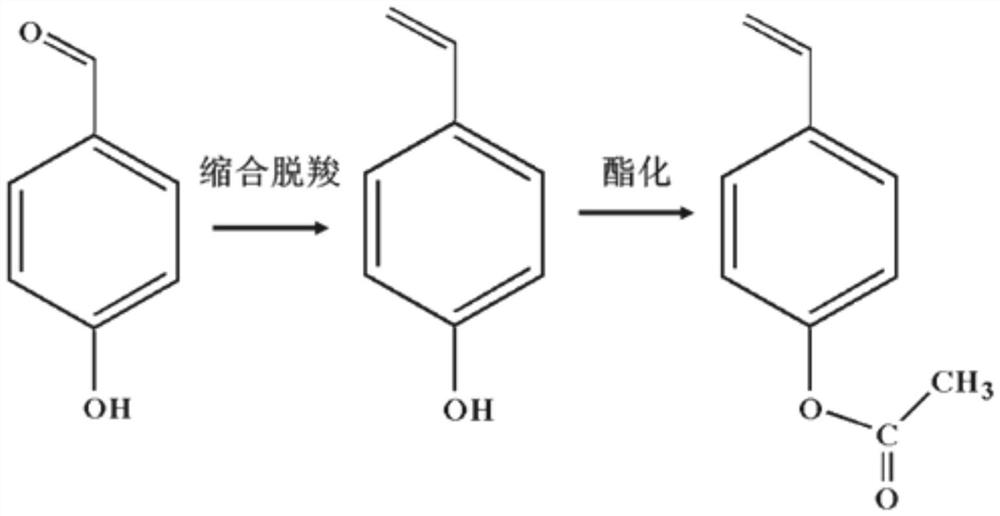
Method for preparing p-acetoxystyrene by one-pot method - Eureka | Patsnap develop intelligence library